
The German version is legally binding (german).
Zusammensetzung
Composition
Dexamethasone.
Excipients
Dexamethason Galepharm 1 mg tablets
Lactose monohydrate 73 mg, microcrystalline cellulose, croscarmellose sodium, equivalent to a maximum of 0.2 mg sodium, magnesium stearate.
Dexamethason Galepharm 4 mg tablets
Lactose monohydrate 70 mg, microcrystalline cellulose, croscarmellose sodium, equivalent to a maximum of 0.2 mg sodium, magnesium stearate.
Pharmaceutical form and active substance quantity per unit
Divisible tablets with 1 mg or 4 mg dexamethasone.
Indications/Uses
Cerebral oedema (induced by cerebral tumour, traumatic brain injury, intracerebral bleeding, neurosurgical interventions, cerebral abscess, encephalitis, meningitis, radiation damage). Acute episodes of chronic polyarthritis that cannot be adequately managed by non-steroidal anti‑inflammatory drugs. Severe bronchial asthma. Acute erythroderma. Acute episodes of pemphigus vulgaris, initial treatment of acute uncontrollable eczema. Acute episodes of cutaneous sarcoidosis and ulcerative colitis. Severe infectious diseases (only in conjunction with antibiotics). Palliative treatment of malignant tumours. Prophylaxis and treatment of cytostatic-induced vomiting.
Treatment of coronavirus disease 2019 (COVID-19) in adult and adolescent patients (aged 12 years and older with body weight at least 40 kg) who require supplemental oxygen therapy.
Dosage/Administration
Dexamethasone is administered at an individual dosage, taking into account the severity and progression of the disease, the patient’s response, and the anticipated duration of treatment.
The daily dosage should be administered in the morning as a single dose (circadian therapy). When treating cerebral oedema or as part of palliative and antiemetic therapy, administration of the daily dosage in 2-4 individual doses may be required.
If needed, the Dexamethason Galepharm tablets can be halved. They should be swallowed whole (not chewed) after meals with some liquid.
To reduce undesirable effects, an initial high dosage should be reduced gradually (at intervals of a few days) as soon as the patient’s condition allows. The maintenance dose for dexamethasone is usually no more than 0.5-1 mg per day.
For cerebral oedema, treatment of severe cases is usually initiated with intravenous administration of dexamethasone, with a switch to oral treatment at a dosage of 4-16 mg daily as the condition improves. In milder cases, oral administration of 2-8 mg may be sufficient.
For acute episodes of chronic polyarthritis that cannot be adequately managed by non-steroidal anti‑inflammatory drugs, severe bronchial asthma, acute erythroderma, acute episodes of pemphigus vulgaris, cutaneous sarcoidosis and ulcerative colitis, or for the initial treatment of acute uncontrollable eczema, the initial dosage is 4-16 mg per day. For long-term therapy scheduled to follow treatment of the acute episode, treatment should be switched from dexamethasone to predniso(lo)ne.
For severe infectious diseases (only in conjunction with antibiotics), 8-16 mg per day for a total of 2‑3 days with rapid dose tapering.
For the palliative treatment of malignant tumours, 8-16 mg initially; long-term dosage 4‑12 mg per day.
For prophylaxis and treatment of cytostatic-induced vomiting: 8 mg orally on the day before the scheduled cytostatic therapy; 8-12 mg IV at the start of treatment, then 16-24 mg orally per day for a total of 2 days.
Dose linearity of the 1 mg tablets in relation to the 4 mg tablets has not been studied.
For the treatment of Covid-19, adult patients receive 6 mg PO, once a day for up to 10 days. Duration of treatment should be guided by clinical response and individual patient requirements.
Long-term treatment
As the stress response is impaired with long-term glucocorticoid therapy, adjustment of the corticosteroid dose in the event of stressful conditions is necessary. For this, hydrocortisone IV is usually used:
- For systemic diseases: Doubling or, if necessary, trebling of the last Dexamethason Galepharm dosage administered.
- For minor surgery: Additional 100 mg hydrocortisone IV before the start
- For moderate surgery: Additional 100 mg hydrocortisone IV prior to surgery and then 100 mg hydrocortisone every 6 hours for 24 hours.
- For major operative surgery: Additional 100 mg hydrocortisone before the start of surgery and then every 6 hours for at least 72 hours. Further treatment depending on course.
As with all glucocorticoid therapy, treatment with Dexamethason Galepharm should not be discontinued abruptly, but rather terminated by slow tapering of the daily dose so as to avoid exacerbation or acute recurrence of the disease, acute adrenocortical insufficiency and cortisone withdrawal syndrome.
If Dexamethason Galepharm is to be used instead of other glucocorticoids, the equivalent doses should be taken into account (see “Properties/Effects”).
Special dosage instructions
Children and adolescents
The dosage should be titrated more to the severity of the disease and response to therapy than to age, body weight or height. In infants and children aged 0‑11 years, lower doses than in adults are generally sufficient. Following an adequate response, the dexamethasone dose should be reduced in small decrements to the lowest possible dose and discontinued as soon as possible. During long‑term treatment, therapy should be intermittent or on an alternate-day basis.
For severe conditions, IV administration of dexamethasone is required in most cases (see information for professionals of Dexamethason Galepharm Amp). If a switch to oral medication is possible, posology is the same as the IV dosage. The following general dosing recommendations apply:
- Acute episode of chronic polyarthritis that cannot be adequately managed by non-steroidal anti-inflammatory drugs:
0.15-0.45 mg/kg per day (in life-threatening situations: a short infusion with 3 mg/kg dexamethasone).
- Severe bronchial asthma:
0.15-0.3 mg/kg per day for a few days; 0.03‑0.04 mg/kg per day over the longer-term.
- Acute episodes of severe dermatosis (e.g. erythroderma, pemphigus vulgaris, uncontrollable eczema, cutaneous sarcoidosis):
The dosage depends on severity: high dosage 0.3-0.45 mg/kg per day, medium dosage 0.15 mg/kg per day.
- Prophylaxis and treatment of cytostatic-induced vomiting:
2-4 mg about 30 minutes prior to cytostatic administration, plus 2-4 mg about 6-8 hours after chemotherapy.
Treatment of Covid-19
Elderly
No dose adjustment is needed.
Renal impairment
No dose adjustment is needed.
Hepatic impairment
No dose adjustment is needed.
Paediatric population
Paediatric patients (adolescents aged 12 years and older) are recommended to take 6mg/dose PO once a day for up to 10 days. Duration of treatment should be guided by clinical response and individual patient requirements. There are only limited data available for patients aged less than 18 years.
Contraindications
This medicinal product must not be used in case of hypersensitivity to any of the ingredients.
Warnings and precautions
Possible complications during corticosteroid therapy are dependent on the dosage level and the duration of therapy. Therefore, the benefits and risks regarding dosage and duration of treatment should be individually assessed for each patient. It should also be established whether daily or intermittent therapy is indicated.
Long-term treatment should be performed only after careful evaluation of the benefits and risks, with patients carefully monitored for signs necessitating a reduction in dosage or discontinuation of the medicine.
Long-term treatment beyond 2 weeks can lead to adrenocortical insufficiency due to inhibition of ACTH release, which may progress to adrenocortical atrophy. Failure of adrenocortical function may last for up to one year or more and signifies a life-threatening risk for the patient in stressful and pressure situations.
As with all glucocorticoid therapy, treatment with Dexamethason Galepharm should not be discontinued abruptly, but rather terminated by slow tapering of the daily dosage so as to avoid exacerbation or acute recurrence of the disease, acute adrenocortical insufficiency and cortisone withdrawal syndrome.
In acute cerebral oedema and acute severe asthma, Dexamethason Galepharm should not be administered as a replacement for conventional treatment, but rather as an adjuvant to such treatment.
Glucocorticoids should not be used in patients with uncomplicated chronic respiratory disease.
Treatment with Dexamethason Galepharm can induce immunosuppression and thereby increase the risk of bacterial, viral, fungal, parasitic and opportunistic infections. Furthermore, Dexamethason Galepharm can mask the symptoms of an existing or developing infection and hence complicate diagnosis. Latent infections, including tuberculosis or hepatitis B, may be reactivated.
In the palliative treatment of malignant tumours, glucocorticoids are generally administered in addition to tumour-specific therapy.
Systemic corticosteroids should not be stopped for Covid-19 patients who are already treated with systemic (oral) corticosteroids for other reasons (e.g. patients with chronic obstructive pulmonary disease) but not requiring supplemental oxygen.
Caution is required in the following cases:
- Acute viral infections (hepatitis B, varicella, herpes zoster, herpes simplex, herpetic keratitis, poliomyelitis, measles): Particular caution is advised if immunosuppressed patients with no history of varicella or measles infection come into contact with persons with measles or chickenpox during Dexamethason Galepharm therapy. The course of these diseases can be particularly severe in patients receiving Dexamethason Galepharm therapy. Varicella infections that occur during systemic treatment with corticosteroids may run a severe course and may ultimately be fatal, especially in children. They require immediate treatment, e.g. with aciclovir IV. In patients at risk, prophylaxis with aciclovir or passive immunoprophylaxis with varicella-zoster immunoglobulin is indicated;
- Acute and chronic bacterial infections: Use only with antibiotic protection. Latent amoebiasis must be excluded prior to treatment, and patients with latent tuberculosis or organ tuberculosis must be given tuberculostatic agents prophylactically during long-term treatment with glucocorticoids;
- Systemic fungal and parasitic infections. In patients with known or suspected Strongyloides infestation, glucocorticoids may result in recurrence or spread of the disease;
- Approximately 8 weeks before and up to 2 weeks after immunisation with live vaccines. Immunisation with inactivated vaccines is theoretically possible. However, it should be remembered that the immune response and hence successful vaccination may be compromised at higher glucocorticoid dosages;
- Lymphadenitis after BCG vaccination;
- HBsAg-positive, chronic hepatitis;
- Hard-to-control diabetes mellitus, as glucose tolerance may be reduced; regular blood sugar monitoring should be performed and, if necessary, the antidiabetic dose should be adjusted;
- Hypothyroidism and liver cirrhosis, as the effect of glucocorticoids is potentiated in such cases;
- Thrombotic tendency;
- Acute myocardial infarction;
- Hard-to-control hypertension and heart failure;
- Myasthenia gravis and co-administration of cholinesterase inhibitors, as the effect of cholinesterase inhibitors in such cases is reduced and the risk of myasthenic crisis is increased (cholinesterase inhibitors should, whenever possible, be discontinued 24 hours prior to administering a corticosteroid);
- Gastrointestinal ulcers;
- Osteoporosis;
- Psychiatric disorders including suicidality (including a history thereof): neurological or psychiatric monitoring is recommended;
- Narrow- and open-angle glaucoma, corneal ulcers or lesions: close ophthalmologic monitoring and therapy are recommended.
Due to the risk of intestinal perforation, Dexamethason Galepharm may be used only when strictly indicated, together with appropriate monitoring, in patients with:
- Severe ulcerative colitis with imminent perforation;
- Diverticulitis;
- Intestinal anastomosis (immediately postoperatively).
The symptoms of peritoneal irritation after gastrointestinal perforation may be absent in patients receiving high glucocorticoid doses.
If fluoroquinolones are co-administered, there is an increased risk for the onset of tendinopathy, tendovaginitis and tendon rupture.
During situations of physical stress, a temporary increase in the daily corticosteroid dose may be required.
Severe anaphylactic reactions may occur, requiring appropriate precautions (readiness to treat anaphylactic shock).
At high doses, adequate potassium intake and sodium restriction must be ensured. Serum potassium levels must also be monitored.
At high doses, bradycardia may occur.
Following use of dexamethasone alone or in combination with chemotherapeutic agents, tumour lysis syndrome (TLS) has been reported in patients with malignant haematological disorders. Patients with tumours with a high proliferation rate or high sensitivity to cytostatic agents, as well as patients with a high tumour burden, are at high risk of developing TLS and should therefore be monitored closely. Appropriate precautions should also be taken.
Pheochromocytoma crisis
Pheochromocytoma crisis, which can be fatal, has been reported after administration of systemic corticosteroids. Corticosteroids should only be administered to patients with suspected or identified pheochromocytoma after an appropriate risk/benefit evaluation.
If a patient under treatment with corticosteroids presents potential symptoms of pheochromocytoma crisis, such as hypertensive crisis, cardiac failure, tachycardia, headache, abdominal pain, and/or chest pain, the possibility of a previously unknown pheochromocytoma should be considered.
Visual disturbances
Visual disturbances can occur during systemic or topical treatment with corticosteroids. If symptoms occur such as blurred vision or other visual disturbances, the patient should be referred to an ophthalmologist to clarify possible causes, such as cataracts, glaucoma or rare diseases such as central serous chorioretinopathy, which have occurred during treatment with systemic or topical corticosteroids.
Premature infants
After early therapy (<96 hours post partum) in premature infants with chronic lung disease with starting doses of 0.25 mg/kg twice daily, available data indicate negative long-term effects with regard to neuronal development.
Children and adolescents
During the growth phase of children, Dexamethason Galepharm should be used only after careful consideration of the benefits and risks. Either intermittent or alternate-day therapy should be performed.
Elderly patients
As elderly patients are at increased risk of osteoporosis, Dexamethason Galepharm should be used only after careful assessment of the benefits and risks.
Excipients
Dexamethason Galepharm contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Dexamethason Galepharm.
This medicinal product contains less than 1 mmol sodium (23 mg) in each tablet, i.e. it is almost ‘sodium-free’.
Interactions
| Medicinal product | Change in effect |
| Saluretics, amphotericin, laxatives | Additional potassium excretion |
| Medicines that induce CYP3A4, such as rifampicin, phenytoin, carbamazepine, barbiturates and primidone | Reduction in the corticosteroid effect due to increased clearance |
| Medicines that inhibit CYP3A4, such as ketoconazole, itraconazole, ritonavir and cobicistat | Risk of systemic corticosteroid side effects may be increased |
| Ephedrine | Reduction in effect due to accelerated corticosteroid metabolism |
| Non-steroidal anti-inflammatory drugs/antirheumatics | Risk of gastrointestinal bleeding, ulceration and perforation increased |
| Salicylates | Reduction in the salicylate effect due to increased clearance. In long-term treatment, increased risk of gastrointestinal bleeding and ulceration that may progress to perforation. The glucocorticoid dose should be cautiously reduced, as salicylate intoxication may occur. |
| Oestrogens | Potentiation of the corticosteroid effect possible due to reduced clearance |
| Immunization with inactivated vaccines | Adverse effect on immunization possible |
| Cholinesterase inhibitors | Reduced cholinesterase inhibition |
| Cardiac glycosides | Glycoside effect potentiated as the result of potassium deficiency |
| Oral antidiabetics, insulin | Blood sugar decrease reduced, possible increased need for antidiabetics |
| Coumarin derivatives | Anticoagulant effect attenuated or potentiated; adjustment of the anticoagulant dose may be necessary during concomitant use |
| Praziquantel | Possible reduction in praziquantel concentrations in the blood |
| Atropine and other anticholinergics | Risk of an additional increase in already elevated intraocular pressure |
| ACE inhibitors | Increased risk for the onset of blood dyscrasias |
| Chloroquine, hydroxychloroquine, mefloquine | Increased risk for the onset of myopathies and cardiomyopathies |
| Immunosuppressants
| Increased susceptibility to infections and possible manifestation or exacerbation of existing latent infections |
| Ciclosporin | In addition, there is an increased risk for the onset of cerebral seizures due to an increase in ciclosporin blood levels |
| Non-depolarising muscle relaxants | Muscle relaxation possibly prolonged |
| Somatropin | Possible reduction in the effect of somatropin during long-term therapy |
| Protirelin | Less pronounced TSH increase possible |
| Fluoroquinolones | Possibly increased risk for the onset of tendinopathy |
| Antacids | If taken concomitantly: possible reduction in glucocorticoid bioavailability and hence reduced efficacy |
Pregnancy, lactation
Pregnancy
Animal studies have shown undesirable effects on the foetus; no controlled human studies exist. As with all glucocorticoids, dexamethasone crosses the placental barrier. Hence, for instance, intrauterine growth disturbances cannot be excluded in the event of long-term therapy during pregnancy. With treatment at the end of pregnancy, there is a foetal risk of adrenocortical atrophy, which may require tapered replacement therapy in the neonate.
Dexamethason Galepharm, like all glucocorticoids, should therefore not be used during pregnancy – and especially in the first three months – unless it is clearly necessary. If an indication exists, prednisolone (or prednisone) should be preferred over all other glucocorticoids, especially fluorinated glucocorticoids, as it has the least ability to cross the placenta.
A physician must always be notified if pregnancy occurs or is suspected.
Children whose mothers have been treated with higher glucocorticoid doses during pregnancy should be carefully monitored for signs of hypoadrenocorticism.
Lactation
Like all other glucocorticoids, dexamethasone is excreted in human milk in very small amounts and might adversely affect adrenocortical function, infant growth, etc. Mothers receiving glucocorticoids during lactation should therefore discontinue breastfeeding.
Effects on ability to drive and use machines
Particularly at the start of treatment with Dexamethason Galepharm, changes in the ability to drive and use machines may occur. In particular, such impairment is due to changes in mood, motivation and the ability to concentrate.
Undesirable effects
The undesirable effects of dexamethasone are dependent on the dosage and duration of treatment, as well as the patient’s age, sex and underlying disease.
The risk of undesirable effects is low with short-term corticosteroid therapy. However, vigilance is required for intestinal bleeding (often stress-related), possibly with few symptoms, as a result of corticosteroid use.
With longer-term, high-dose therapy, the known undesirable effects of glucocorticoids may occur.
Infections and infestations
Increased risk of infection, masking of infections, onset, exacerbation or reactivation of bacterial, viral, fungal, parasitic and opportunistic infections, activation of strongyloidiasis.
Blood and lymphatic system disorders
Moderate leukocytosis, lymphopenia, eosinopenia, polycythaemia.
Immune system disorders
Hypersensitivity reactions, serious anaphylactic reactions such as arrhythmia, bronchospasm, blood pressure increase or decrease, circulatory failure, cardiac arrest.
Endocrine disorders
Adrenal insufficiency.
Development of a pheochromocytoma crisis in patients with pre-existing (even latent) pheochromocytoma.
Cushing’s syndrome (e.g. moon face, truncal obesity); see “Warnings and precautions”.
Metabolism and nutrition disorders
Sodium retention with oedema formation, increased potassium, calcium and phosphate excretion.
Weight gain, reduced glucose tolerance, diabetes mellitus, hypercholesterolaemia, hypertriglyceridaemia, increased appetite.
Psychiatric disorders
Psychosis, mania, depression, hallucinations, labile mood, irritability, increased drive, euphoria, inner restlessness, sleep disorders, suicidality.
Nervous system disorders
Increased intracranial pressure with papilloedema (pseudotumor cerebri), onset or exacerbation of epilepsy (seizures).
Eye disorders
Increase in intraocular pressure (glaucoma), lens opacity (cataract), especially with posterior subcapsular lens opacity, exacerbation of corneal ulcer symptoms, promotion of viral, fungal and bacterial eye infections, exacerbation of bacterial corneal infections, ptosis, mydriasis, chemosis, iatrogenic scleral perforation, chorioretinopathy, blurred vision.
Vascular disorders
Hypertension.
Increased risk of arteriosclerosis and thrombosis, vasculitis, increased capillary fragility.
Gastrointestinal disorders
Peptic stomach and duodenal ulcers, gastric bleeding, pancreatitis, gastric complaints.
Skin and subcutaneous tissue disorders
Stretch marks (striae rubrae), perioral dermatitis, skin atrophy, pinpoint bleeding under the skin (petechiae), bruising (ecchymosis), steroid acne, delayed wound healing, telangiectasia, hypertrichosis, changes in skin pigmentation.
Musculoskeletal and connective tissue disorders
Muscular atrophy, muscle weakness, myopathy, tendinopathy, tendovaginitis, tendon rupture, osteoporosis, aseptic osteonecrosis, growth retardation in children, epidural lipomatosis.
Reproductive system and breast disorders
Disturbances of sex hormone secretion (absence of menstrual bleeding, abnormal hair growth, impotence).
Reporting suspected adverse reactions after authorisation of the medicinal product is very important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions online via the ElViS portal (Electronic Vigilance System). You can obtain information about this at www.swissmedic.ch.
Overdose
As the acute toxicity of glucocorticoids is low, cases of intoxication caused by an acute glucocorticoid overdose have thus far been observed only on rare occasions. Increased undesirable effects are to be expected in cases of chronic overdose, particularly affecting the endocrine system, metabolism and electrolyte balance. There is no specific antidote in the event of an overdose, and treatment is symptomatic.
Properties/Effects
ATC code
H02AB02
Mechanism of action
The mechanism of action of glucocorticoids is multifaceted.
Dexamethasone binds to and activates intracellular receptors. The activated glucocorticoid receptor complex migrates to the cell nucleus, where it initiates or blocks the synthesis of certain proteins at specific DNA binding sites.
- Among the proteins whose synthesis is initiated is lipocortin 1, which inhibits phospholipase A2 which is important for an inflammatory response, and angiotensin-converting enzyme (ACE), which intervenes in blood pressure regulation.
- Among the proteins whose synthesis is inhibited are various cytokines (e.g. TNF alpha, interleukin-2, interleukin-6), which activate the cells of the immune system, and various pro‑inflammatory enzymes (e.g. collagenase). Also inhibited is the induction of NO synthetase and cyclooxygenase.
In addition to these genomic mechanisms, which start with a latency of 30 minutes up to several hours, there are a few rapid effects, some of which start even at low plasma concentrations (e.g. suppression of endogenous cortisol secretion), as well as others that become effective only at higher concentrations (e.g. membrane stabilisation). The most likely mechanism for the latter is the incorporation of glucocorticoids into the cell membrane as an initial event.
Pharmacodynamics
Dexamethasone has a potent anti-inflammatory, anti-allergic (anti-oedematous) and immunosuppressive effect. It increases carbohydrate metabolism, is non-specifically antitoxic (membrane protection) and stimulates the microcirculation (stabilisation of cerebral perfusion).
Dexamethasone has a very low mineralocorticoid effect.
The relative anti-inflammatory equivalent dose of dexamethasone compared to other glucocorticoids is 1 mg dexamethasone = 6 mg triamcinolone or methylprednisolone = 7.5 mg prednisone or prednisolone = 30 mg hydrocortisone = 35 mg cortisone.
Clinical efficacy
Like other glucocorticoids, dexamethasone is used for a wide range of indications. Due to its long biological half-life, dexamethasone is particularly suitable for such indications where a continuous glucocorticoid effect is desired. In some indications, dexamethasone is preferred due to the low mineralocorticoid effect.
For the treatment of Covid-19
The RECOVERY trial (Randomised Evaluation of COVid-19 thERapY) is an investigator-initiated, individually randomised, controlled, open-label, adaptive platform trial to evaluate the effects of potential treatments in patients hospitalised with COVID-19.
The trial was conducted at 176 hospital organizations in the United Kingdom.
There were 6425 Patients randomised to receive either dexamethasone (2104 patients) or usual care alone (4321 patients). 89% of the patients had laboratory-confirmed SARS-CoV-2 infection.
At randomization, 16% of patients were receiving invasive mechanical ventilation or extracorporeal membrane oxygenation, 60% were receiving oxygen only (with or without non-invasive ventilation), and 24% were receiving neither.
The mean age of patients was 66.1+/-15.7 years. 36% of the patients were female. 24% of patients had a history of diabetes, 27% of heart disease and 21% of chronic lung disease.
Primary endpoint
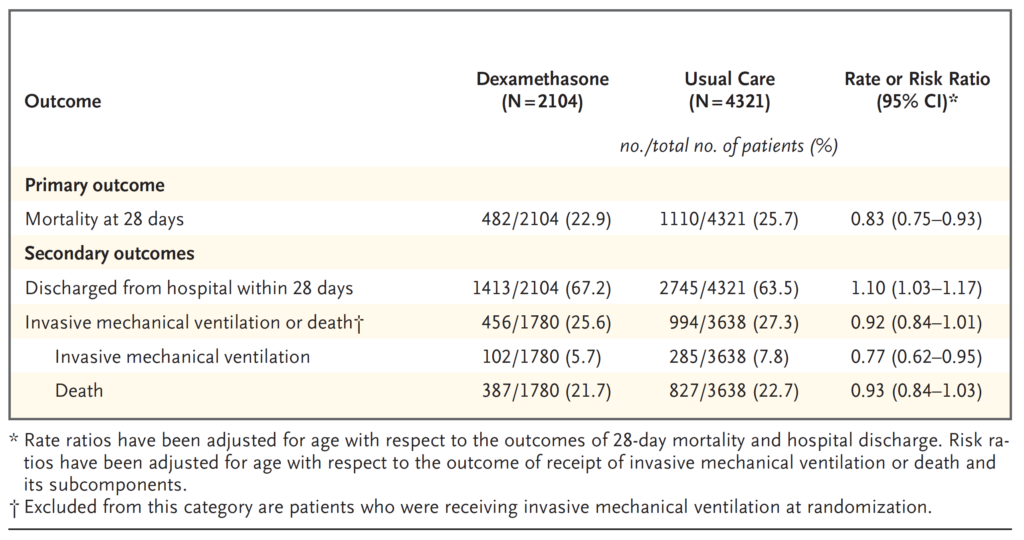
Mortality at 28 days was significantly lower in the dexamethasone group than in the usual care group, with deaths reported in 482 of 2104 patients (22.9%) and in 1110 of 4321 patients (25.7%), respectively (rate ratio, 0.83; 95% confidence interval [CI], 0.75 to 0.93; P<0.001).
In the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and in those receiving supplementary oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94).
There was no clear effect of dexamethasone among patients who were not receiving any respiratory support at randomization (17.8% vs. 14.0%; rate ratio, 1.19; 95% CI, 0.91 to 1.55).
Secondary endpoints
Patients in the dexamethasone group had a shorter duration of hospitalization than those in the usual care group (median, 12 days vs. 13 days) and a greater probability of discharge alive within 28 days (rate ratio, 1.10; 95% CI, 1.03 to 1.17).
In line with the primary endpoint the greatest effect regarding discharge within 28 days was seen among patients who were receiving invasive mechanical ventilation at randomization (rate ratio, 1.48; 95% CI, 1.16 to 1.90), followed by oxygen only (rate ratio, 1.15; 95% CI, 1.06 to 1.24) with no beneficial effect in patients not receiving oxygen (rate ratio, 0.96; 95% CI, 0.85 to 1.08).
Safety
There were four serious adverse events (SAEs) related to study treatment: two SAEs of hyperglycaemia, one SAE of steroid-induced psychosis and one SAE of an upper gastrointestinal bleed. All events resolved.
Subgroup analyses
Effects of allocation to Dexamethasone on 28−day mortality, by age and respiratory support received at randomisation
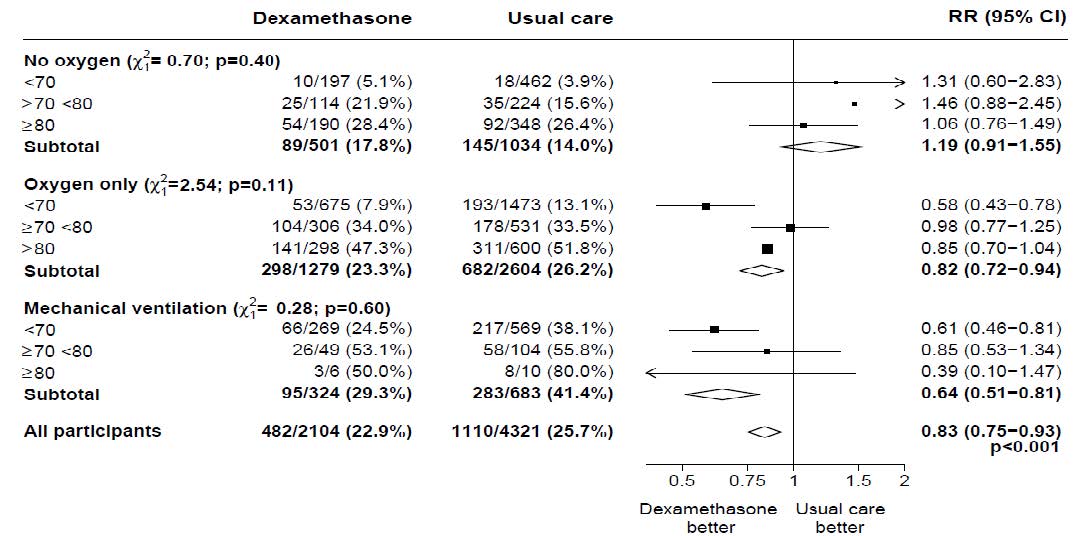
Effects of allocation to Dexamethasone on 28−day mortality, by respiratory support received at randomisation and history of any chronic disease

Pharmacokinetics
Absorption
Following oral administration, dexamethasone is rapidly and completely absorbed in the stomach and upper small intestine. Peak blood levels are reached between the first and second hour. Bioavailability of dexamethasone after oral administration is about 80-90 %. However, the peak pharmacological effect is not reached until 6-24 hours post-administration. Dose linearity of the 1 mg tablets in relation to the 4 mg tablets has not been studied.
Distribution
Depending on the dose, about 80 % of dexamethasone is bound to plasma proteins, mainly plasma albumin. Within the very high dose range, the major fraction circulates freely, i.e. not bound to protein, in the blood. The volume of distribution for dexamethasone is 0.6-0.8 L/kg. In patients with hypoalbuminaemia, the fraction of unbound (active) corticosteroid increases.
Dexamethasone crosses both the blood-brain and placental barrier, and it passes into breast milk.
Metabolism
Dexamethasone is mainly excreted unchanged via the kidneys. In humans, only a small fraction of molecules is hydrated or hydroxylated, resulting in 6-hydroxydexamethasone and 20‑dihydrodexamethasone as main metabolites. 30-40 % of dexamethasone molecules are bound in the human liver to glucuronic acid or sulphuric acid and appears in this form in the urine.
Elimination
The plasma elimination half-life of dexamethasone is 3-5 hours, whilst the biological half-life at 36‑72 hours is considerably longer. Plasma clearance in adults is 2-5 mL/min/kg.
Kinetics in specific patient groups
During pregnancy, the elimination half-life is prolonged.
Hepatic impairment
In cases of severe hepatic disease (e.g. hepatitis, liver cirrhosis) or hypothyroidism, the elimination half-life is prolonged.
Renal impairment
Renal impairment does not significantly affect elimination.
Children and adolescents
In newborn infants, plasma clearance is lower than in children and adults.
Preclinical data
Glucocorticoids have very low acute toxicity.
Long-term toxicity (or repeat dose toxicity)
There are no available data on chronic toxicity in humans or animals.
Mutagenicity
Dexamethasone has not been sufficiently investigated for mutagenic effects. There are provisional indications of a mutagenic potential, the relevance of which is not yet clear.
Carcinogenicity
No long-term animal studies are available.
Reproductive toxicity
In animal experiments, dexamethasone induces cleft palate in mice, rats, hamsters, rabbits and dogs, as well as other malformations to a minor extent.
Other information
Effects on diagnostic methods
Allergy tests
Skin reactions may be suppressed.
Blood serum levels
Decreased: ESR, clotting time (Lee White), uric acid, testosterone, potassium, TSH, thyroxin, T3.
Increased: glucose, cholesterol, sodium, chloride.
Urine parameters
Decreased: 17-ketosteroids.
Increased: creatinine, calcium, glucose (in predisposed patients).
Shelf life
Do not use this medicine after the expiry date (“EXP”) stated on the container.
Special precautions for storage
Do not store above 30 °C. Store in the original packaging in order to protect the contents from light.
Keep out of the reach of children.
Authorisation number
57974 (Swissmedic).
Packs
Dexamethason Galepharm 1 mg, divisible tablets: Packs of 20 and 100 tablets. (B)
Dexamethason Galepharm 4 mg, divisible tablets: Packs of 20 and 100 tablets. (B)
Marketing authorisation holder
Galepharm AG, Zurich.
Date of revision of the text
January 2022.